The changing paradigm – away from a one-size-fits-all approach towards precision medicine – raises the importance of comprehensive genomic profiling of tumor samples. Comprehensive genomic tumor profiling refers to the simultaneous evaluation of several biomarkers within one analysis. This approach not only enables detecting the most relevant predictive markers for current targeted therapies but also key immuno-oncology biomarkers, such as the tumor mutational burden (TMB) and the microsatellite instability (MSI). TMB is a biomarker that measures the number of somatic mutations present in a cancer patient’s tumor and is quantified as mutations per megabase (mut/Mb). Another key immunotherapy biomarker is the MSI, which is caused by the failure of the DNA mismatch repair system.
The application areas of comprehensive tumor profiling are manifold, with a focus on:
- stratifying patients for the best treatment choice
- identifying patients eligible for clinical trials
- driving clinical research, especially in the area of immune therapy
You can choose between different products to perform comprehensive tumor profiling.
CeGaT Is the Best Partner for Sequencing Your Project
Our Commitment to You
Fast Processing
Turnaround time
≤ 15 business days
High Quality
Highest accuracy for all processes
Secure Delivery
Secure provision of sequenced data via in-house servers
Safe Storage
Safe storage of samples and data after project completion
Our Service
We provide a comprehensive and first-class project support – from selecting the appropriate product to evaluating the data. Each project is supervised by a committed scientist. You will have a contact person throughout the whole project.
Our service includes:
- detailed project consulting
- product selection tailored to your project
- detailed bioinformatic evaluation of your data
- detailed project report with information about sample quality, sequencing parameters, bioinformatic analysis, and results
Benefit from our dedicated support and accredited workflows.
Explore Our Product Portfolio for Comprehensive Tumor Profiling
We offer different Comprehensive Tumor Profiling (CTP) products to address a variety of research questions. Our CTP Somatic and CTP Tumor are based on the enrichment of our Whole Exome Sequencing (WES) products. They are processed analogously to WES by using the DRAGEN Bio-IT platform. These products offer insights into more than 20,000 genes. For CTP Somatic, a normal and a tumor sample are required for a tumor-normal comparison. For CTP Tumor, we only require a tumor sample.
If you are interested in only a subset of genes relevant for profiling a tumor, we offer different Panel Sequencing products within our Comprehensive Tumor Profiling product portfolio. When choosing our CTP TUM Panel, more than 760 cancer-associated genes are covered in this panel. Furthermore, 31 therapy-relevant fusions are analyzed at a high sequencing depth. Our CTP PAT Panel covers 55 genes highly relevant to tumor diseases. In addition, we offer the CTP FUS Panel. In contrast to the other two DNA panels, the CTP FUS Panel is an RNA panel focusing on fusions relevant to tumor diseases.
Would you like to have bioinformatic analyses performed on your data in addition to the included deliverables? Each of our products can be supplemented with further services. We are happy to advise you.
CTP Somatic | CTP Tumor | CTP TUM Panel | CTP PAT Panel | CTP FUS Panel |
Species | Species | Species | Species | Species |
Sequencing panel | Sequencing panel | Sequencing panel | Sequencing panel | Sequencing panel |
Number of analyzed genes | Number of analyzed genes | Number of analyzed genes | Number of analyzed genes | Number of analyzed genes |
Analysis of tumor and normal tissue | Analysis of tumor and normal tissue | Analysis of tumor and normal tissue | Analysis of tumor and normal tissue | Analysis of tumor and normal tissue |
Starting material | Starting material | Starting material | Starting material | Starting material |
Sequencing platform | Sequencing platform | Sequencing platform | Sequencing platform | Sequencing platform |
Output | Output (dependent on the tumor content) | Output | Output | Output |
Included deliverables | Included deliverables | Included deliverables | Included deliverables | Included deliverables |
If you are interested in sequencing panels not related to tumor profiling, please have a look at our Panel Sequencing product portfolio.
Bioinformatics
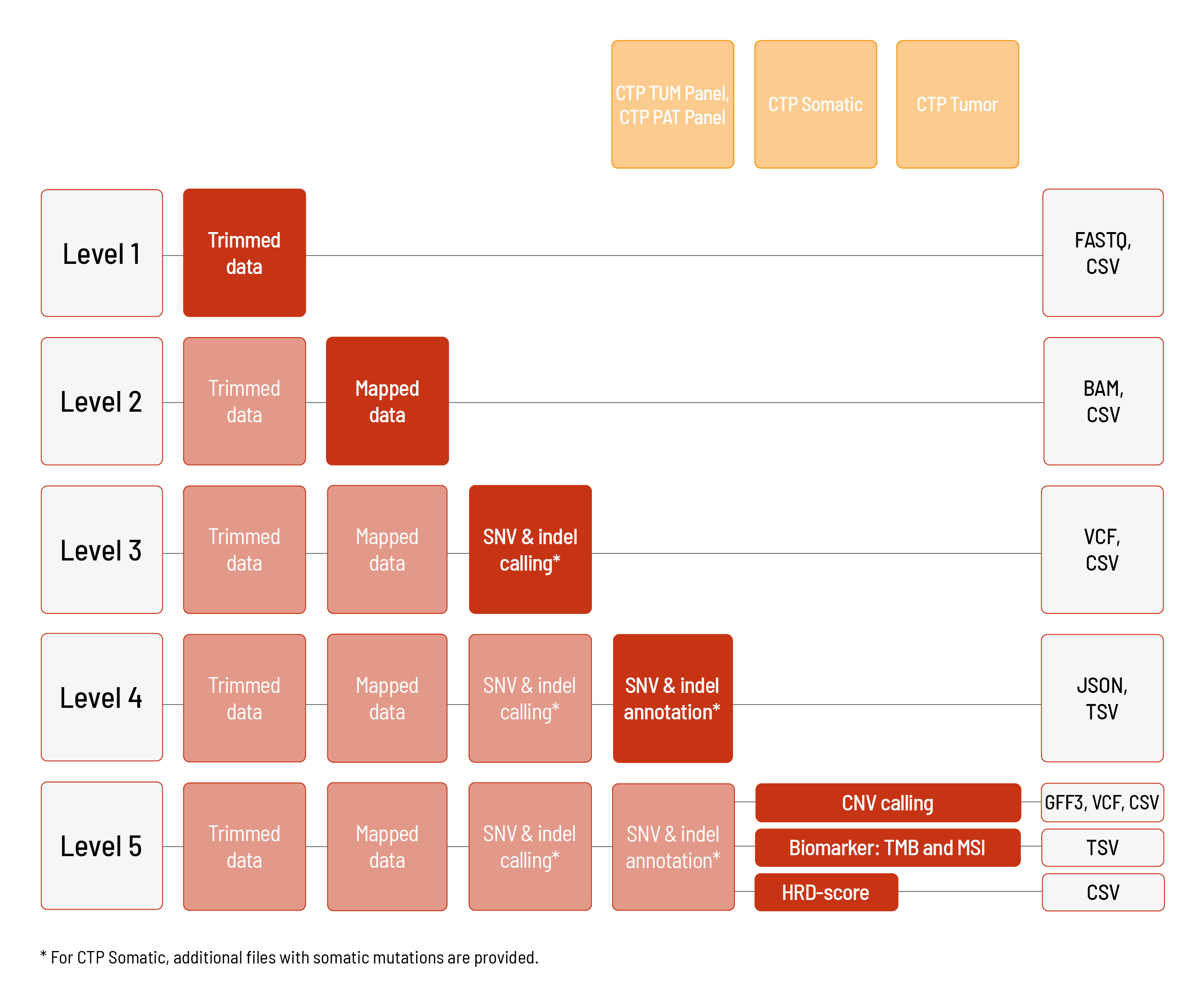
Raw sequencing data are automatically processed. We offer different levels of bioinformatic analysis. The default level is Level 1. With increasing bioinformatic level, more data are delivered. All higher levels include the data from the lower levels. In addition to the data, and independent of the analysis level, a project report is generated.
Analyses for CTP Somatic, CTP Tumor, CTP TUM Panel, and CTP PAT Panel:
We can perform the analyses for CTP Somatic and CTP Tumor based on the human references hg19 or GRCh38. The analyses for CTP TUM Panel and CTP PAT Panel are based on the human reference hg19 and are available until level 4.
Level 1:
- demultiplexing and adapter trimming of the sequencing data
(FASTQ format) - metrices (CSV format)
- MultiQC report (HTML format)
Level 2:
- mapping of the sequencing data (BAM format)
- metrices (CSV format)
Level 3:
- calling of single nucleotide variants (SNVs) and small insertions and deletions (indels) (VCF format)
- metrices (CSV format)
Level 4:
- annotation of the SNVs and indels (JSON and TSV format)
Level 5 (one of the following):
- calling of copy number variations (CNVs) (VCF and GFF3 format) including metrices (CSV format)
- biomarker determination: TMB and MSI (TSV format)
- calculation of HRD-Score (CSV format) (only for CTP Somatic)
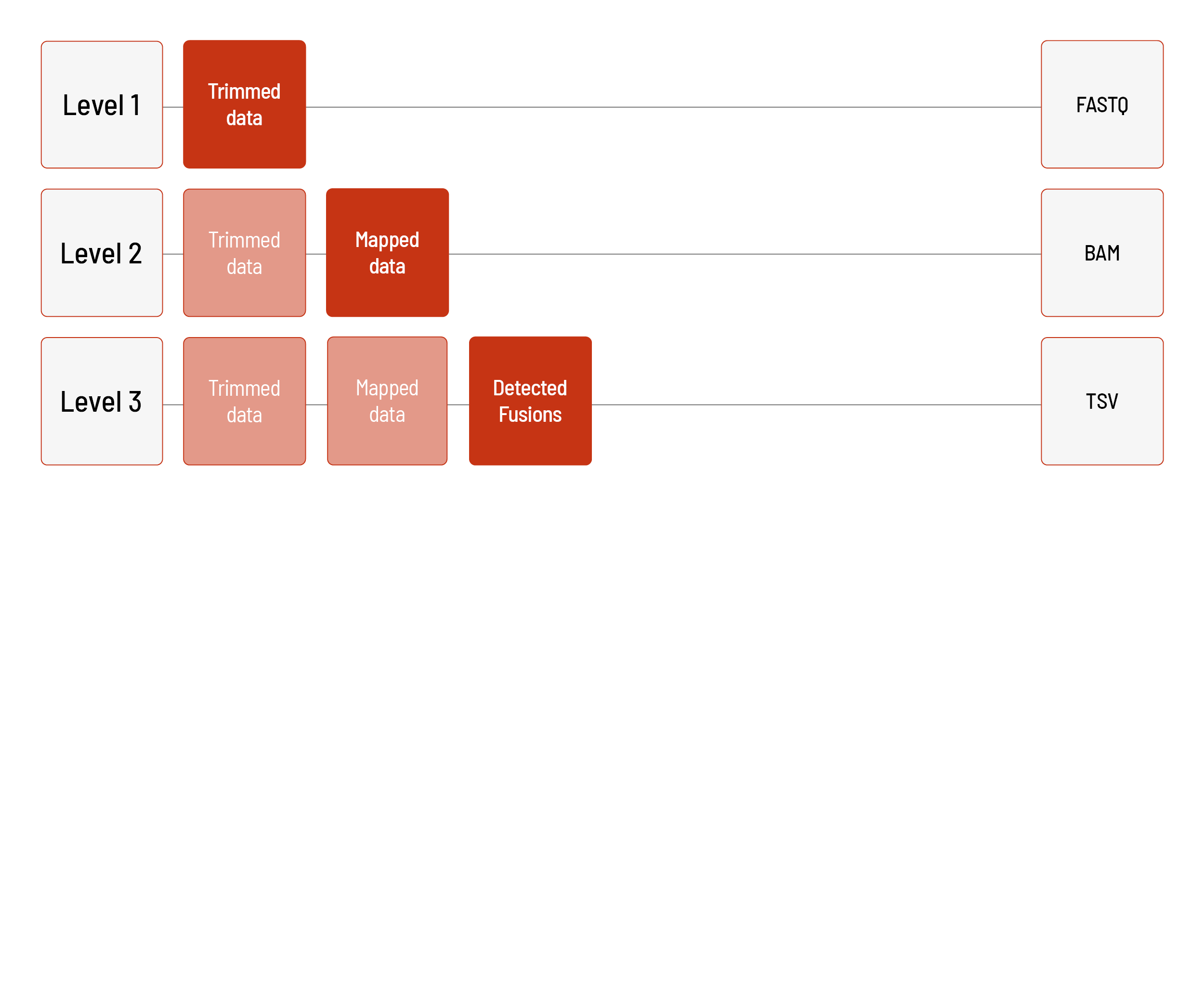
Analyses of CTP FUS Panel:
We can perform the analyses for CTP FUS Panel based on the human reference genome hg19.
Level 1:
- demultiplexing and adapter trimming of the sequencing data
(FASTQ format)
Level 2:
- mapping of the sequencing data (BAM format)
Level 3:
- delivery of detected fusion events (TSV format)
Technical Information
At CeGaT, paired-end sequencing (2 x 100 bp) is performed using the Illumina sequencing platforms. If you require other sequencing parameters, please let us know! We can provide further solutions.
Gene Directory for CTP TUM Panel, CTP PAT Panel, and CTP FUS Panel
Further Information about Comprehensive Tumor Profiling
The tumor mutational burden, also known as TMB, is the amount of gene mutations occurring in a patient’s tumor, but not occurring in the patient’s healthy tissue. Thus, these mutations are coding, somatic mutations. The tumor mutational burden is defined as mutations per megabases (mut/Mb). It should not be confused with the variant allele frequency that only indicates the frequency of an allele. In contrast, the tumor mutational burden is the total amount of mutations per megabases in a sample. Depending on the tumor entity, the tumor mutational burden varies: Some tumors do not show high mutation rates, such as the Ewing sarcoma or acute myeloid leukemia. Other cancer types, such as melanomas or lung squamous cell carcinomas, hold a high tumor mutational burden. Thus, the tumor can be classified as low, intermediate, or highly mutated using the tumor mutational burden. The tumor mutational burden is a promising predictive immunotherapeutic biomarker, for example in immune checkpoint inhibitor therapy. Such an immune therapy is especially effective in patients with a high tumor mutational burden. A higher number of tumor-specific mutations leads to a higher number of altered proteins, so-called neoantigens. These neoantigens are different from the healthy proteins, resulting in an increased difference of the tumor tissue compared to the healthy tissue.
Both tumor and healthy cells present parts of their protein content on their cell surface. These presented protein parts are also called peptides. Immune cells regularly check the peptides on the surfaces of the proteins. If the peptides are recognized as foreign, the immune system is activated. The tumor peptides, the neoantigens, are foreign to the immune system. Thus, a higher tumor mutational burden correlates with more mutations and more neoantigens that are recognized as foreign by the immune system. With a higher tumor mutational burden, the immune system is activated more easily.
Another immunotherapeutic biomarker is the microsatellite instability, also known as MSI. The microsatellite instability indicates the number of mutations in satellite regions. These satellite regions are special DNA repeat sequences. The satellite regions of the DNA are the main component of the functional centromeres, forming the main structure of the heterochromatin. A disturbed DNA mismatch repair mechanisms (MMR) can lead to genetic hypermutability in the satellite regions, resulting in microsatellite instability. The microsatellite instability is classified as stable for a low MSI score, or unstable for a high MSI score.
Downloads
Contact Us
Do you have a question or are you interested in our service? Feel free to contact us. We will take care of your request as soon as possible.
Start Your Project with Us
We are happy to discuss sequencing options and to find a solution specifically tailored to your clinical study or research project.
When getting in contact, please specify sample information including starting material, number of samples, preferred library preparation option, preferred sequencing depth and required bioinformatic analysis level, if possible.